New FDA approved drug to treat Alzheimer's only helps in early stages, experts say
FOX 2 - The Food and Drug Administration has approved a new drug to fight Alzheimer's. The drug, Aducanumab, is the first new treatment in nearly 20 years.
It is also the first that works to attack this disease process - instead of only treating symptoms of dementia.

FDA approves new drug in battle against Alzheimer's
The Food and Drug Administration has approved a new drug to fight Alzheimer's. The drug, Aducanumab, is the first new treatment in nearly 20 years.
Jennifer Lepard is Alzheimer's Association Michigan Chapter and is excited at the news saying the diagnosis of mild cognitive impairment now comes with hope.
"This drug really targets people with MCI or mild cognitive impairment or early-stage Alzheimer's," she said. "And my relative falls into that category."
The treatment works like this:
"Imagine an IV in someone's arm and it is being infused into your system, so this is the same thing," said Dr. Stewart Graham. "What it does is slowing the progression of the disease, it is targeting the disease."
Researchers and doctors say the treatment is promising, but they do have concerns.
"This drug only treats early stages of Alzheimer's disease," said Graham, director of Alzheimer's disease research at Beaumont. "So we don't know how it works on people with later stages of this disease."
The drug also comes with a hefty price tag. According to the drug maker's website, the yearly cost at the maintenance dose would be $56,000.
"Is Medicare and Medicaid going to cover the cost?" said Graham.
Researchers at Beaumont are working to learn even more about this treatment.
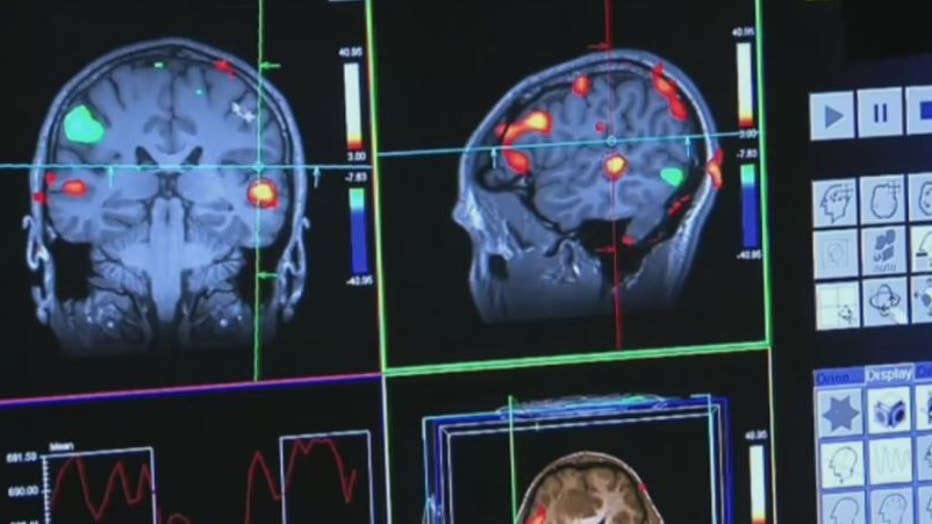
"Here at Beaumont we're going to be one of the sites where we're going to look at this drug and individuals with more advanced stages of Alzheimer's disease, to see if it has any impact," he said.
But for the nearly 200,000 people living in Michigan with this disease, this treatment is a sign of the medical advancements they've been waiting for, and their advocates say it's also a push for why early diagnosis is critical.
For more information: www.alz.org/gmc

